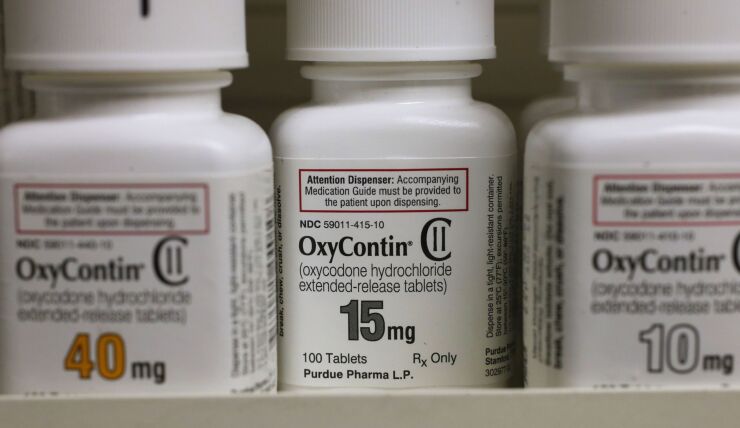
McKinsey has agreed to pay $573 million to settle claims by U.S. states that the consulting company helped fuel the opioid epidemic by providing marketing advice to drugmakers including Purdue Pharma and Johnson & Johnson.
New York-based McKinsey will also produce tens of thousands of its internal documents detailing its work for Purdue Pharma and other opioid companies for public disclosure online, according to a statement by North Carolina Attorney General Josh Stein, who helped lead negotiations on behalf of dozens of U.S. states.
Read more:
“We allege that McKinsey helped to develop and promote schemes that led to widespread overprescription of Purdue Pharma’s OxyContin,” Stein said on Thursday.
McKinsey will pay about 80% of the money immediately to beef up treatment programs and bolster police budgets strained by
The consulting firm suggested ways to “turbocharge” sales of Purdue’s
“McKinsey continues to cooperate with government agencies on matters relating to our past work for opioid manufacturers, and we will not be commenting further at this time,” a company spokesman said Wednesday in an emailed statement. McKinsey is the 35th-largest private company in the U.S. with $10.5 billion in 2019 revenue, Forbes estimates. It has offices in more than 130 cities and 65 countries, according to its website.
Read more:
More than 3,000 state and local governments have targeted opioid makers and distributors in hopes of recouping billions in tax dollars spent dealing with the fallout of the U.S. opioid epidemic. More than 400,000 Americans have died over the last two decades from overdoses tied to opioid-based drugs. Many of the municipalities’ suits have been consolidated before a federal judge in Cleveland.
In December, McKinsey officials issued a
McKinsey said in
Stamford, Connecticut-based Purdue sought
In Purdue’s bankruptcy court records and its Justice Department settlement, the government said McKinsey consultants suggested in 2017 that the drugmaker compensate insurers if a covered consumer became addicted or overdosed up to a limit of $14,000 per patient. Purdue never set up such a program.
A McKinsey partner, in an unsealed 2019 email disclosed in court filings, told a colleague they should give the consulting firm’s risk managers a head’s up about their Purdue work. “It probably makes sense to have a quick conversation with the risk committee to see if we should be doing anything other that [sic] eliminating all our documents and emails,” the partner wrote.
In its December apology, McKinsey said it was “undertaking a full review of the work in question, including into the 2018 email exchange which referenced potential deletion of documents.”